For an updated version of these pages, click here

Substitution preferences:
All protein types:
| Favoured | Arg ( 2) | Glu ( 1) | Gln ( 1) | |||||
| Neutral | Ser ( 0) | Asn ( 0) | ||||||
| Disfavoured | Met (-1) | Ala (-1) | Asp (-1) | Pro (-1) | Thr (-1) | His (-1) | Val (-2) | Leu (-2) |
| Tyr (-2) | Gly (-2) | Cys (-3) | Trp (-3) | Ile (-3) | Phe (-3) |
| Favoured | Arg ( 1) | |||||||
| Neutral | Asp ( 0) | Glu ( 0) | Asn ( 0) | Gly ( 0) | His ( 0) | Met ( 0) | Pro ( 0) | Gln ( 0) |
| Ala ( 0) | Ser ( 0) | Thr ( 0) | ||||||
| Disfavoured | Cys (-1) | Ile (-1) | Val (-1) | Trp (-1) | Tyr (-1) | Leu (-1) | Phe (-2) |
| Favoured | Arg ( 1) | |||||||
| Neutral | Gln ( 0) | Pro ( 0) | Asp ( 0) | Glu ( 0) | Asn ( 0) | His ( 0) | Ile ( 0) | Ala ( 0) |
| Ser ( 0) | Thr ( 0) | Val ( 0) | ||||||
| Disfavoured | Met (-1) | Leu (-1) | Gly (-1) | Tyr (-1) | Phe (-2) | Trp (-2) | Cys (-6) |
| Favoured | Arg ( 9) | Gln ( 6) | Asn ( 5) | His ( 4) | Asp ( 3) | Trp ( 3) | Glu ( 1) | Tyr ( 1) |
| Disfavoured | Met (-1) | Ser (-1) | Gly (-1) | Ala (-2) | Thr (-2) | Cys (-3) | Val (-4) | Ile (-4) |
| Leu (-4) | Pro (-4) | Phe (-5) |
Role in structure: Lysine frequently plays an important role in structure. First, it can be considered to be somewhat amphipathic as the part of the side chain nearest to the backbone is long, carbon containing and hydrophobic, whereas the end of the side chain is positively charged. For this reason, one can find Lysines where part of the side-chain is buried, and only the charged portion is on the outside of the protein. However, this is by no means always the case, and generally Lysines prefer to be on the outside of proteins.
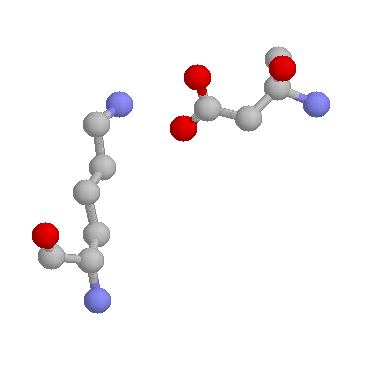
Lysines are also frequently involved in salt-bridges, where they pair with a negatively charged amino acid (such as Aspartate, shown below) to create stabilising hydrogen bonds, that can be important for protein stability.
Lysines are also very often modified, particularly acetylated, which is one of the most common modifications in proteins known.